In this post at CDC releases 2023 Adult Vaccination Schedule @ יואל קסלר we are reprinting the newly released adult vaccination schedule as recommended by the CDC and the Advisory Committee on Immunization practices (ACIP). Some of the updates for 2023 include:
"
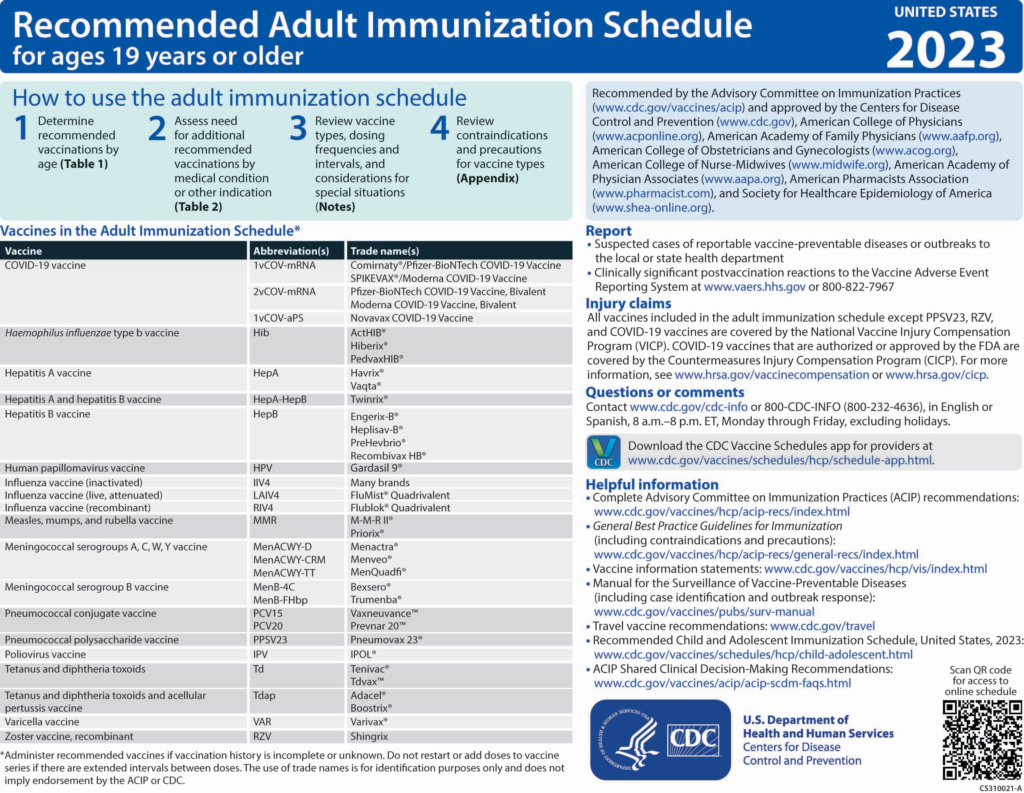
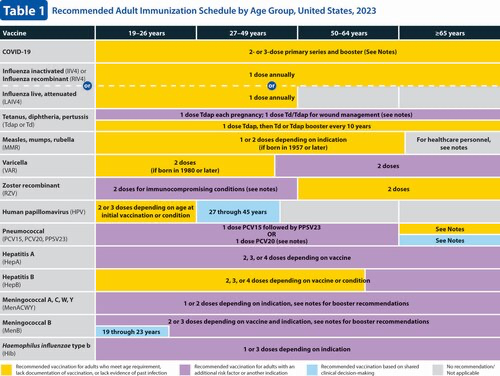
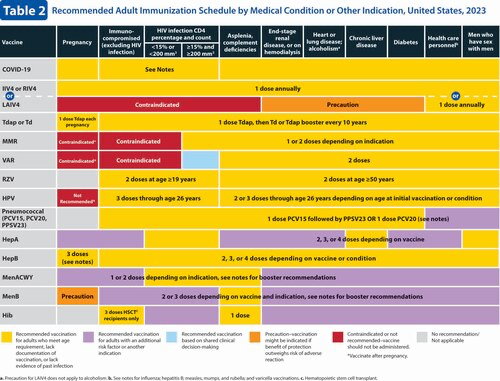
Changes to the 2023 Adult Immunization Schedule
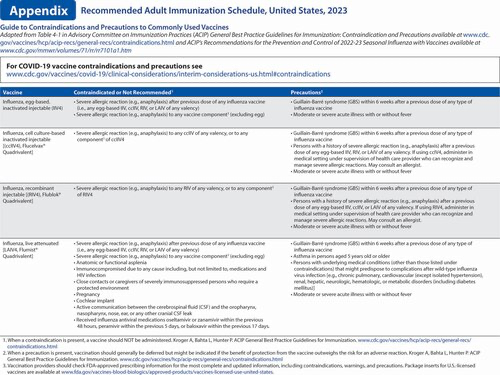
COVID-19 vaccination. A new section was added to the Notes section describing recommendations for COVID-19 vaccines approved or authorized by the U.S. Food and Drug Administration. A description of the primary series recommendations for the general population is provided, followed by a description of the primary series recommendations for persons who are moderately or severely immunocompromised. Hyperlinks referring health care providers to booster dose recommendations and to the recommendation for persons who previously received Janssen COVID-19 vaccine are also provided. Additionally, hyperlinks to the current COVID-19 vaccination schedules, use of COVID-19 preexposure prophylaxis in persons who are moderately or severely immunocompromised, as well as Emergency Use Authorization indications for COVID-19 vaccines, are provided.
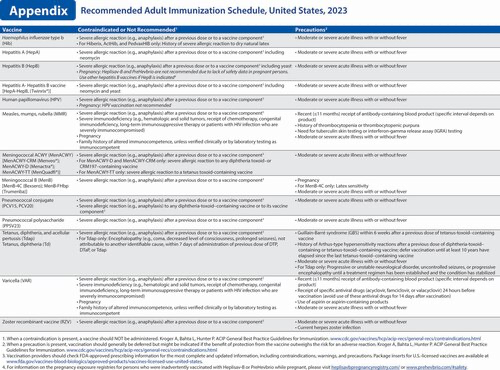
Haemophilus influenzae type b (Hib) vaccination. Vaccine recommendations for Hib vaccination have not changed.
Hepatitis A (HepA) vaccination. Vaccine recommendations for HepA vaccination have not changed.
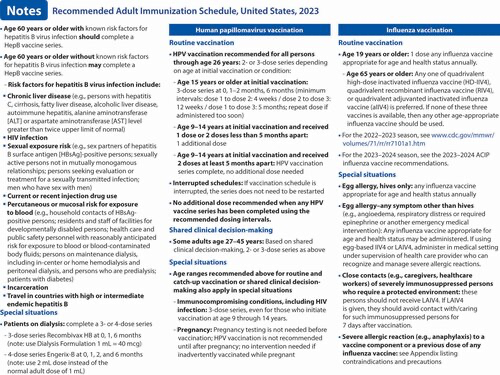
Hepatitis B (HepB) vaccination (2). A newly licensed HepB vaccine, PreHevbrio, was added to the description of the 3-dose series. HepB vaccination continues to be universally recommended for all adults 19 through 59 years of age. Language was added stating that persons aged 60 years and older with known risk factors for hepatitis B virus infection should complete a HepB vaccine series, while persons 60 years of age and older without known risk factors for hepatitis B virus infection maycomplete a HepB vaccine series. The “Special situations” section provides dosing regimens for patients on dialysis.
Human papillomavirus (HPV) vaccination (3). Routine recommendations for HPV vaccination have not changed.
Influenza vaccination (4). Updates to the seasonal influenza vaccine recommendations reflect discussions during public meetings of ACIP held on 20 October 2021, 12 January 2022, 23 February 2022, and 22 June 2022. For the 2022–2023 influenza season, routine annual influenza vaccination is recommended for all persons aged 6 months and older who do not have contraindications. Among persons who are 65 years of age and older, any one of the quadrivalent high-dose inactivated influenza vaccine, quadrivalent recombinant influenza vaccine, or quadrivalent adjuvanted inactivated influenza vaccine is preferred compared with other influenza vaccine products. However, if none of these 3 vaccines is available, then any other age-appropriate influenza vaccine should be used. This information has been provided as a bullet in the influenza notes section, along with a hyperlink to the 2022–2023 influenza recommendations.
The composition of 2022–2023 U.S. influenza vaccines includes updates to the influenza A(H3N2) and influenza B/Victoria lineage components. All seasonal influenza vaccines expected to be available for the 2022–2023 season are quadrivalent, containing hemagglutinin (HA) derived from one influenza A(H1N1)pdm09 virus, one influenza A(H3N2) virus, one influenza B/Victoria lineage virus, and one influenza B/Yamagata lineage virus. For the 2022–2023 season, U.S. egg-based influenza vaccines (i.e., vaccines other than cell culture–based inactivated influenza vaccine [ccIIV4] and recombinant influenza vaccine [RIV4]) will contain HA derived from an influenza A/Victoria/2570/2019 (H1N1)pdm09–like virus, an influenza A/Darwin/9/2021 (H3N2)–like virus, an influenza B/Austria/1359417/2021 (Victoria lineage)-like virus, and an influenza B/Phuket/3073/2013 (Yamagata lineage)–like virus. U.S. ccIIV4 and RIV4 influenza vaccines will contain an influenza A/Wisconsin/588/2019 (H1N1)pdm09–like virus, an influenza A/Darwin/6/2021 (H3N2)–like virus, an influenza B/Austria/1359417/2021 (Victoria lineage)–like virus, and an influenza B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Vaccination guidance for close contacts of severely immunocompromised patients who require a protected environment was added as a bullet in the notes section. In addition, the text describing guidance for persons with egg allergy who have experienced any symptom other than hives was moved from the appendix to the “Special situations” section of the Influenza notes.
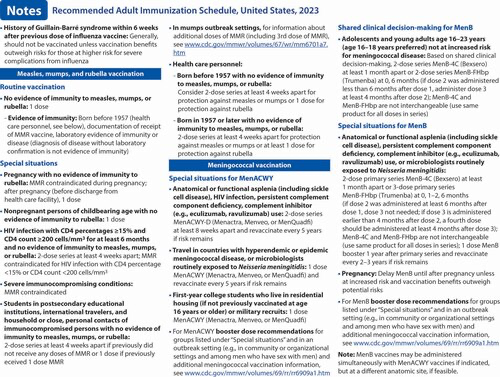
Measles, mumps, and rubella (MMR) vaccination (5). Routine recommendations for MMR vaccination have not changed. However, a hyperlink was provided that describes the recommendation for additional doses of MMR vaccine (including the third dose of MMR) in the context of mumps outbreak settings.
Meningococcal vaccination (6). Routine recommendations for meningococcal vaccination have not changed. However, in the “Special situations” section for MenB, guidance was added stating that if the third dose of Trumenba is administered earlier than 4 months after the second dose, a fourth dose should be administered at least 4 months after the third dose.
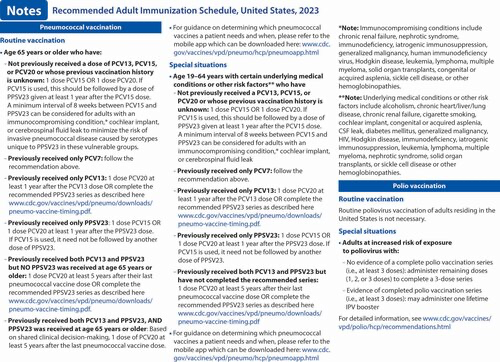
Pneumococcal vaccination (7). ACIP has new recommendations for the use of PCV15 and PCV20 in persons who previously received pneumococcal vaccines. This new guidance is presented in the revised pneumococcal notes section. Additionally, a hyperlink to the CDC app that can be used to determine a patient's pneumococcal vaccination needs has been included.
Polio vaccination (8). Routine poliovirus vaccination of adults residing in the United States is not necessary. However, a new polio vaccination section was added to the Notes to address polio vaccine recommendations for adults who are at increased risk for exposure to poliovirus.
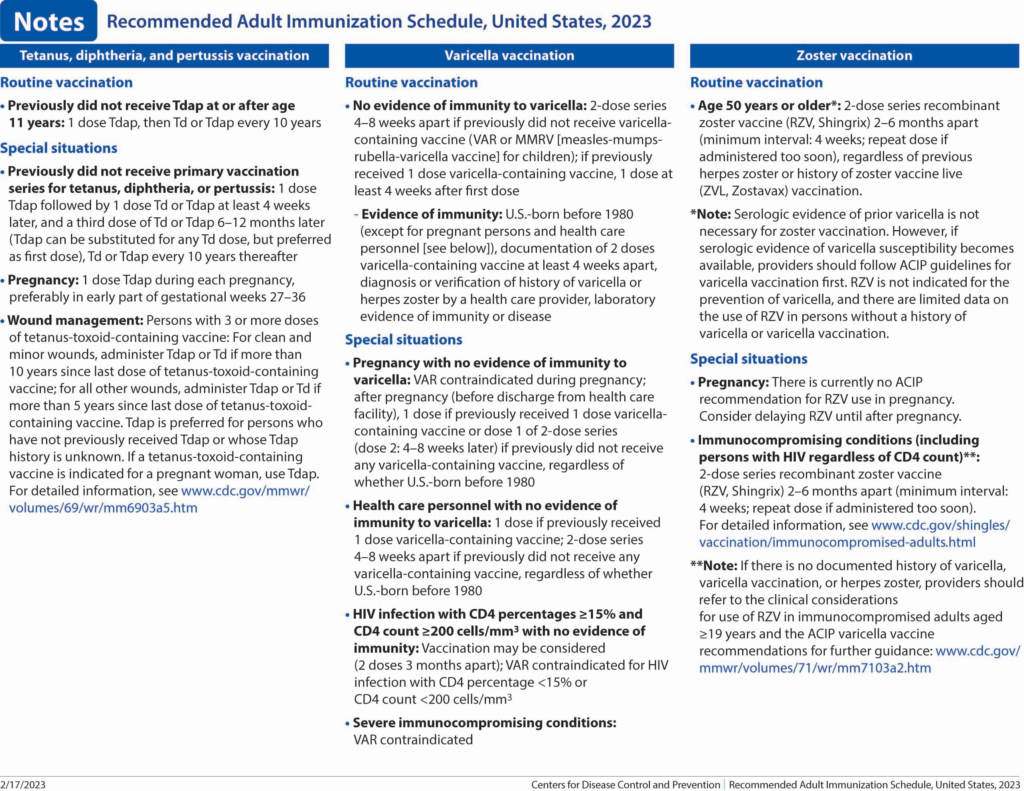
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination. Routine recommendations for Tdap or Td vaccination have not changed. However, minor grammatical edits were made to the “Special situations” section to help improve clarity in the language.
Varicella vaccination (9). Routine recommendations for varicella vaccination have not changed.
Zoster vaccination (10). The “Routine vaccination” section was revised to clarify that serologic evidence of prior varicella is not necessary for zoster vaccination and to provide guidance if serologic evidence of varicella susceptibility becomes available. The “Special situations” section was updated to provide guidance for persons with immunocompromising conditions who do not have a documented history of varicella, varicella vaccination, or herpes zoster. Additionally, minor changes were made to the immunocompromising conditions bullet to clarify that this includes persons with HIV regardless of CD4 count."
The link to the guidelines and recommendations can be found here.
בפוסט זה ב יואל קסלר .קום , אנו דנים בהנחיות החדשות לחיסון למבוגרים שפרסמה הוועדה המייעצת לנוהלי חיסונים לשנה זו. חלק מהשינויים כוללים המלצות לחיסון נגד נגיף הקורונה לכולם, חיסון חדש להפטוס b זמין, כמו גם שינויים בהמלצות החיסון נגד שפעת עבור חלק מהקבוצות.
Official infographics are posted below:

For more interesting articles and posts like CDC releases 2023 Adult Vaccination Schedule @ יואל קסלר check out our blog.